Japanese
Naoki Takeishi
Keywords
Fluid mechanics; computational biomechanics; microcirculation;
(hemo)rheology; capsule; red blood cell (RBC); margination;
leukocyte; platlets; circulating tumor cell (CTC); cell adhesion;
drug delivery; thrombin; tracheal flow; cerebral blood flow;
cerebrospinal fluid flow; oxygen transport; GPGPU
~ Biomechanics of cellular flow and adhesion in microcirculation ~
Cell adhesion to the vascular wall is of crucial importance in both pathological and physiological processes such as leukocyte immune function and cancer metastasis. Cell adhesion is a multiple-step phenomenon, which is initiated by tethering and rolling followed by firm adhesion. This process involves not only the biochemical interaction of adhesion proteins, but also the mechanics of cell and cellular environment. To gain insights into these intravital events, we develop a numerical model of cellular flow and adhesion in microcirculation, and numerically investigate these problems.
Hemorheology
Blood viscosity is a basic biological parameter affecting the blood flow both in large arteries and in microcirculations. Since human blood is a dense suspension consisting of 55% fluid (plasma) and 45% blood cells, with over 98% of the blood cells being red blood cells (RBCs), hydrodynamic interactions of individual RBCs are of fundamental importance for hemorheology. However, much is still unknown, in particular about how the single-cell behaviour relates to the behaviour in suspensions and then rheology. To clarify this question, we perform numerical simulations from dilute to dense suspensions of RBCs.
(More detail is in :Takeishi et al., 2019, J. Fluid Mech.)
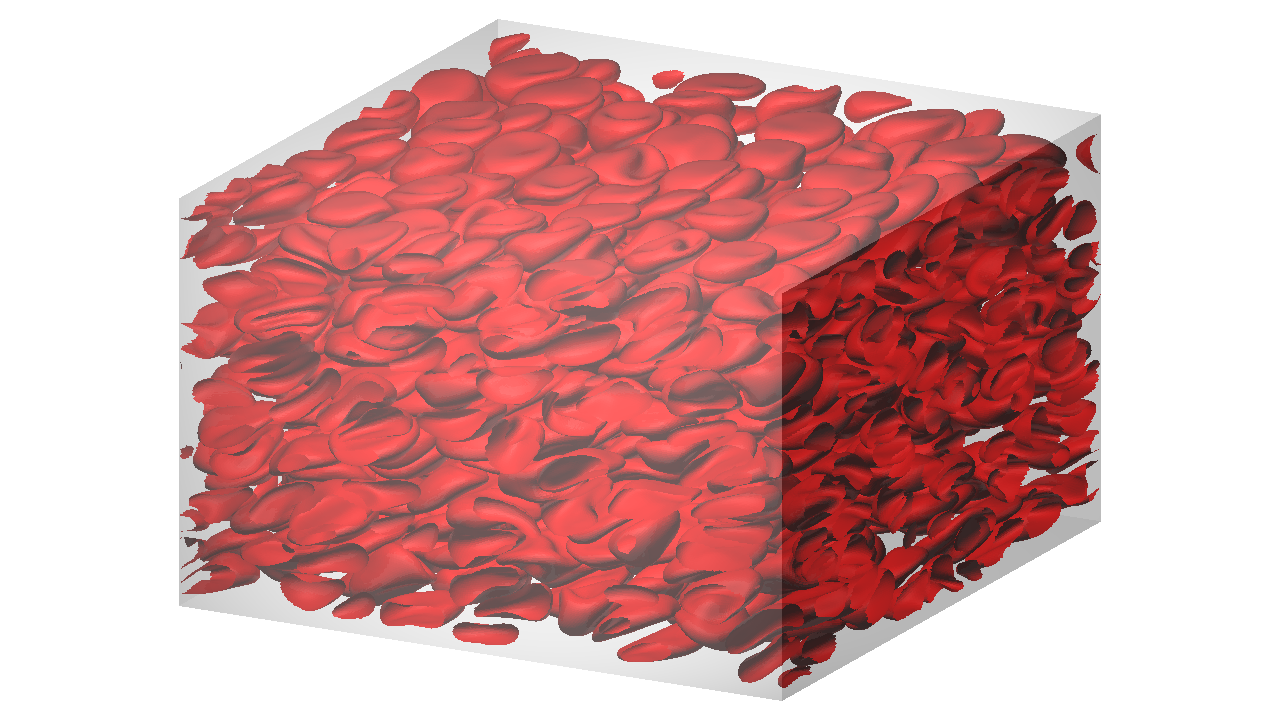
Suspension of RBCs under a shear flow at 41% hematocirt.
Leukocyte margination
A deformable particle flowing in a channel migrates to the center of the channel because of lift forces perpendicular to the wall, induced by the wall and share gradient. Therefore, in microcirculation, RBCs exhibit axial migration. Leukocytes, in contrast to RBCs, appear to flow primarily in the peripheral layer. This is termed margination, which is the first step in the firm adhesion of leukocytes to the endothelium. Our numerical results clearly demonstrated that leukocyte margination occurs due to the passing motion of RBCs for a wide range of channel diameters.
(More detail is in :Takeishi et al., 2014, Physiol Rep.)
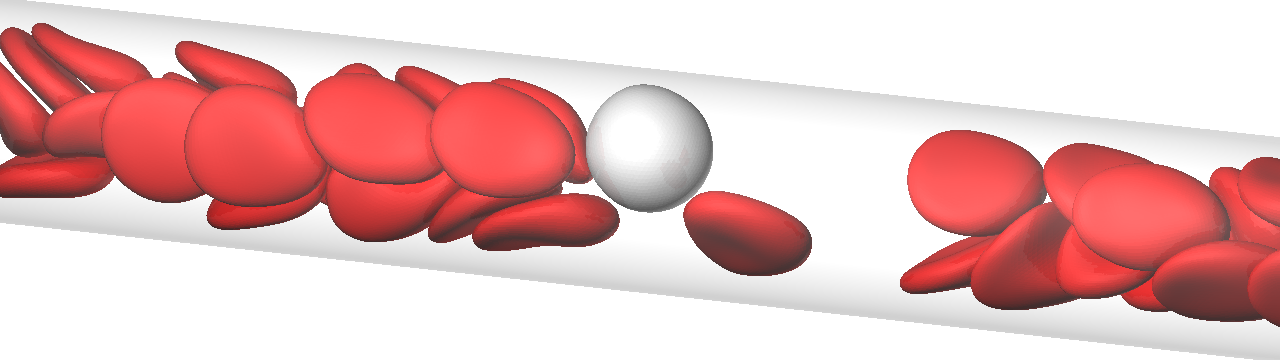
The flow of a leukocyte and RBCs in a microvessel. The flow direction is from left to right.
Flow mode of circulating tumor cell
A total of 90% of cancer-related deaths result from metastasis, whereby tumor cells are shed from a primary tumor and circulate with the flow of blood. Such CTCs adhere to the blood vessels in distant organs, where they form secondary tumors. The metastatic process involves both the solid and fluid mechanics of blood cells, but how CTCs flow with RBCs in the blood remains unclear. We carried out numerical simulations of the flow of a CTC and RBCs in microvessels, and identifiedy similarities and differences between leukocytes and CTCs.
(More detail is in: Takeishi et al., 2015, Phys Rev E. and also in: Inside Science, "Fast, Fluid Cells Spread Cancer")
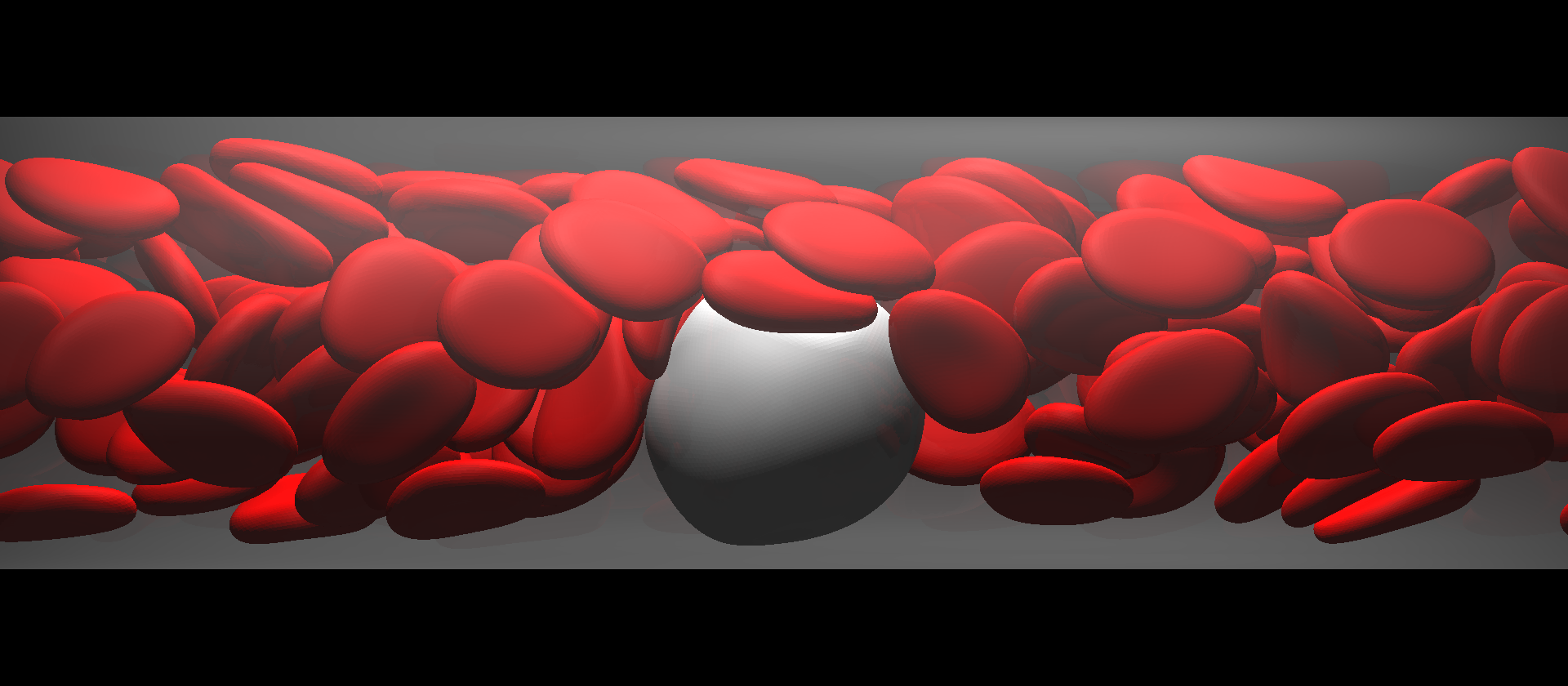
The flow of a CTC and RBCs in a microvessel.
Capture of micropartilces
The question here is whether the behavior of microparticles (MPs) can be formulated in the same context as margination of leukocyte/CTC. In capillaries, the flow mode of RBCs shifted from multi-file flow to bolus (single-file) flow, and MPs were captured by the bolus flow of the RBCs instead of being marginated. In the relatively large microvessel, RBCs formed complete multi-file flow, where most MPs were marginated. Because the flow behavior of MPs is of paramount importance in drug delivery systems targeting capillary districts, these results help us to determine the optimal size of drug carriers.
(More detail is in: Takeishi & Imai, 2017, Sci Rep.)
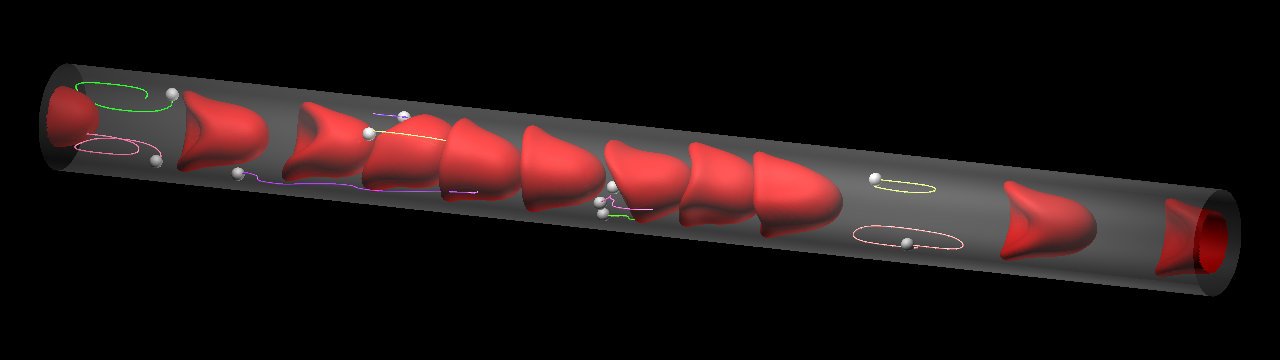
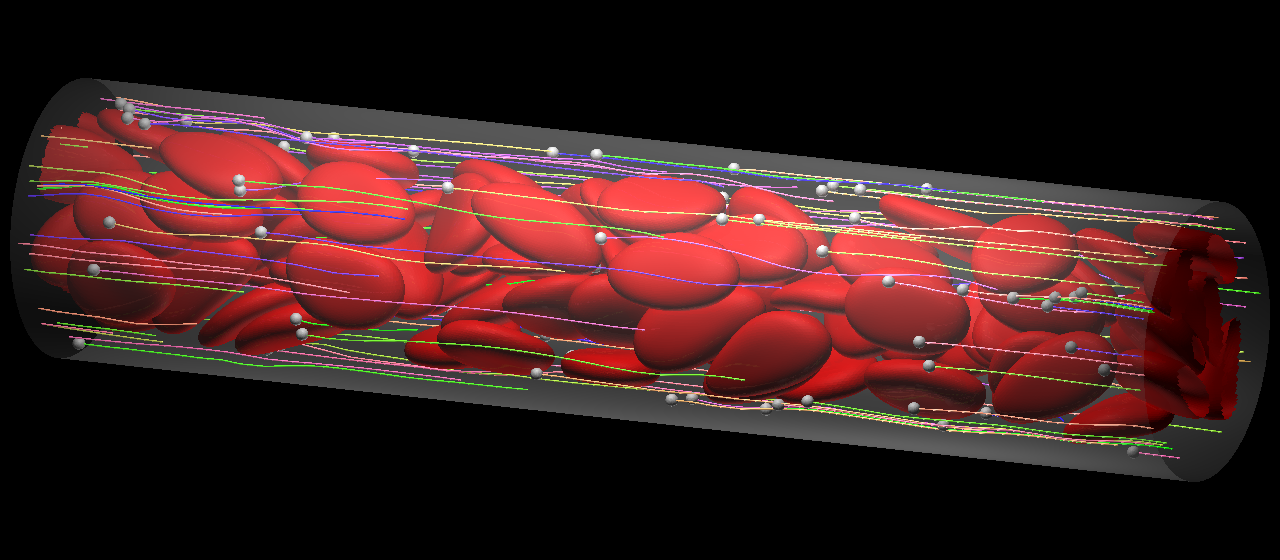
Microparticles are captured by the bolus flow of the RBCs in the capillary (top), while they marginate in the relatively lage microvessel (bottom). Lines show particle trajectories.
Cell adhesion in capillaries
The size of capillaries is often smaller than that of leukocytes and CTCs, and the space is not large enough for cell rolling. If such a size restriction can indeed promote cancer extravasation, CTC adhesion in capillaries is of fundamental importance for understanding cancer metastasis. We focus on cell adhesion in capillaries whose diameter is comparable to or smaller than that of the cell itself. We investigated the behavior of a cell in capillaries under ligand-receptor interactions, and showed that a cell exhibiting "bullet" motion effectively decreases the velocity of the cell.
(More detail is in: Takeishi et al., 2016, Am J Physiol Heart & Circ Physiol.)
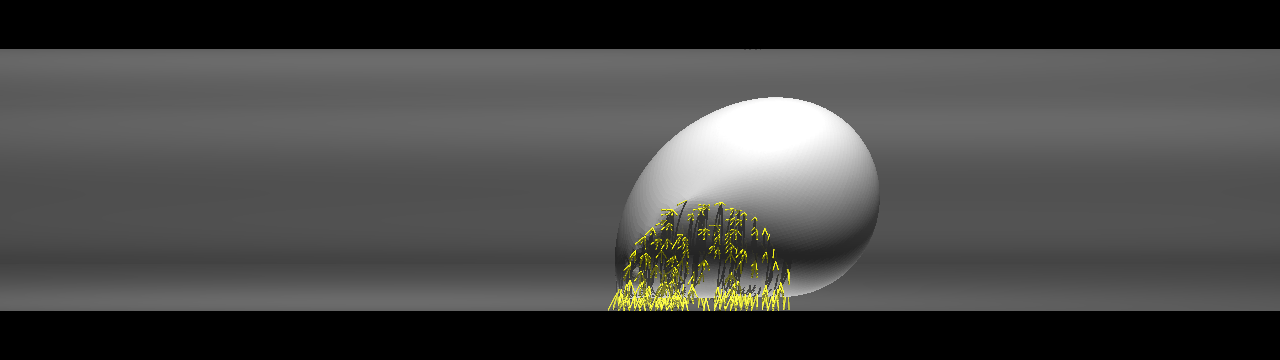
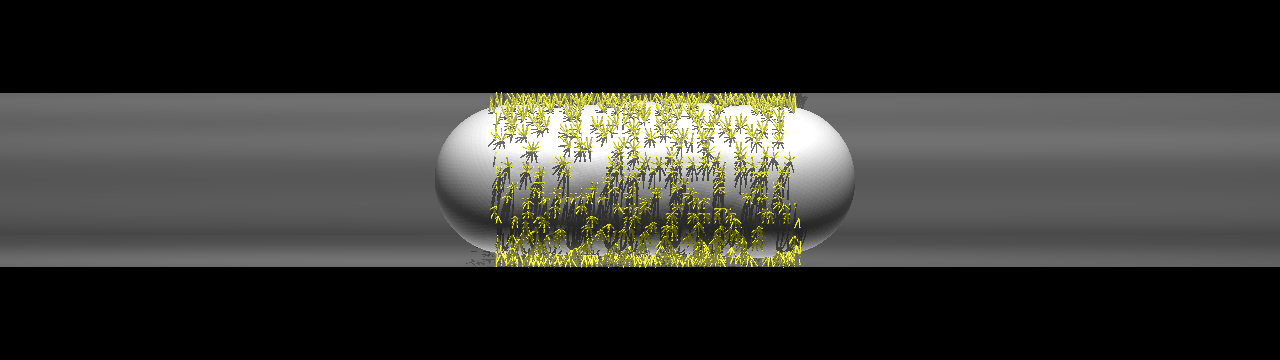
Cell exhibiting a rolling motion (top) in a relatively large capillary and bullet motion (bottom) in a smaller capillary. Ligand-receptor bindings are visualized by small yellow rods.
Copyright © Naoki Takeishi